DNA replication dynamics
Every time a cell divides, it must accurately replicate its entire genetic information and ensure its equal distribution between the two daughter cells. To maintain genomic integrity, DNA replication must be both timely and precise. Errors in this process can lead to mutations, which may kill cells, halt their division, or drive uncontrolled proliferation - a hallmark of cancer.
Our research focuses on the mechanisms that regulate DNA replication speed and genome stability. We investigate how cells balance efficient DNA replication with the prevention of harmful replication errors.
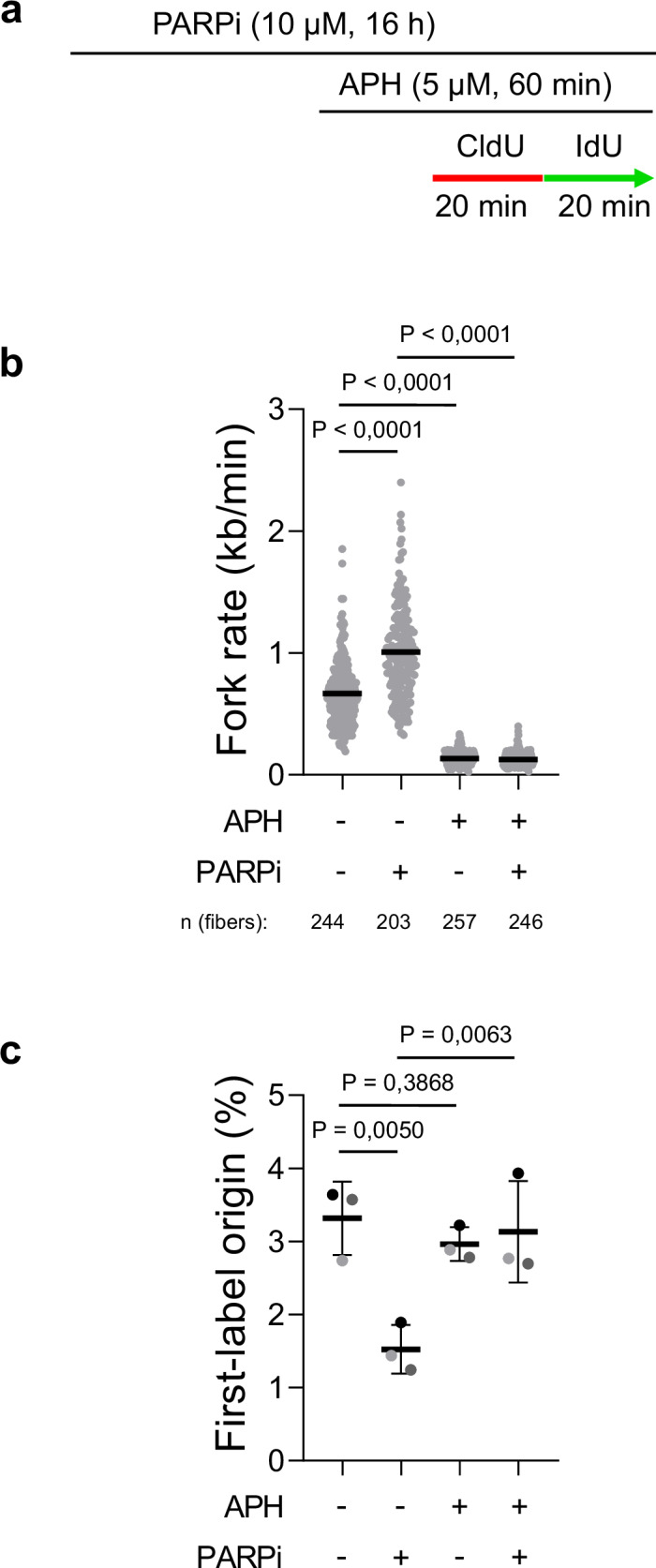
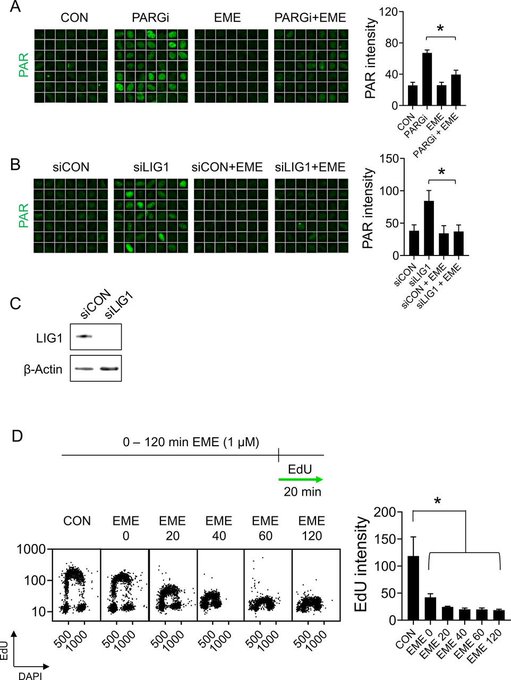
A key area of our work is studying PARP inhibitors, a class of chemotherapy drugs used to treat various cancers, including breast, ovarian, prostate, and pancreatic cancers. We have shown that, rather than stalling or collapsing replication forks as previously thought, PARP inhibitors accelerate DNA replication. However, this increase in replication speed comes at a cost: it is associated with the accumulation of single-stranded DNA gaps, which arise when the replication machinery moves too quickly for proper DNA synthesis and repair. These gaps can compromise genome stability and are likely a key factor in the sensitivity of cancer cells to PARP inhibitor treatment.
By uncovering the molecular mechanisms that control DNA replication dynamics and the effects of PARP inhibition, we aim to advance our understanding of genome maintenance and identify new therapeutic strategies to target cancer cells more effectively.
- DNA replication
- DNA polymerases
- PARP
- Okazaki fragment processing
- Repliction fork reversal
- Replication gap suppression
- DNA polymerases responsible for PARP inhibitor-induced replication fork acceleration
- Okazaki fragment processing and lagging strand synthesis in regulation of replication fork dynamics
- Suppression of fork reversal as a mechanism of PARP inhibitor-induced ieplication fork acceleration
- Single stranded DNA gaps in cancer progression