Juan Bautista De Sanctis, Alexis Hipólito García, Dolores Moreno, Marián Hajduch
Abstract
Coronavirus infections are frequent viral infections in several species. As soon as the severe acute respiratory syndrome (SARS) appeared in the early 2000s, most of the research focused on pulmonary disease. However, disorders in immune response and organ dysfunctions have been documented. Elderly individuals with comorbidities exhibit worse outcomes in all the coronavirus that cause SARS. Disease severity in SARS-CoV-2 infection is related to severe inflammation and tissue injury, and effective immune response against the virus is still under analysis. ACE2 receptor expression and polymorphism, age, gender and immune genetics are factors that also play an essential role in patients' clinical features and immune responses and have been partially discussed. The present report aims to review the physiopathology of SARS-CoV-2 infection and propose new research topics to understand the complex mechanisms of viral infection and viral clearance.
1. Introduction
Coronaviruses (CoVs) are a collection of enveloped viruses with non-segmented, positive-sense single-stranded RNA genomes with distinctive crown-like spikes that protrude from the capsid of helical symmetry. They have a remarkably long RNA genome and a particular replication strategy. In this complex family, several members attack different species causing several diseases that can end up in death. In November 2002, in China, a severe acute respiratory syndrome coronavirus (SARS-CoV) was identified. It promptly spread to other countries. There were around 8000 confirmed cases, and the mortality rate was 9.6%. Then, another member of the family, Middle East respiratory syndrome coronavirus (MERS-CoV), appeared in Saudi Arabia in 2012 and later emerged in South Korea in 2015. The confirmed cases of MERS-CoV exceeded 2000, with a mortality rate of ∼35%. In 2019, another member of the family was identified, SARS-CoV-2.The number of infected people and the mortality rate still grow continuously. Infected elderly individuals with comorbidities exhibit the worst outcomes. There are now several vaccines against SARS-CoV-2 approved for emergency use by the regulatory offices of different countries.
The coronavirus genome is formed by 2 UTR sites, 5′ and 3′, the replicase, the spike (Spike), the envelope E (Envelope), the M (Membrane), the N (Nucleocapsid) and the poly (A) tail. There are additional genes at the end of the genome. The S protein is highly glycosylated, and it is required for infection. Even though the S protein is very similar to SARS (94% nucleotide sequence), a protease‐sensitive site in the SARS‐CoV‐2 is absent in the previous one. The membrane protein and the accessory proteins are non‐essential for replication; however, they have essential viral assembly and pathogenesis roles. Other non‐structural genes, open reading frame (ORF), ORF1ab, ORF3a, ORF6, ORF7a, ORF10 and ORF8, are also transcribed. These proteins' function in the infection, viral replication and host response are still controversial.
The primary transmission is airborne, close and direct human‐to‐human contact, droplets from saliva, sneeze or cough. A less prevalent infection occurs by direct skin contact, faeces or contaminated objects. The incubation period range is from 2 to 14 days, and the infected person can be asymptomatic during this period. SARS‐CoV‐2 has an R0 of 2.2‐2.6, implying that each infected individual has the potential to infect 2.2 other people. One key issue is that the virus is detectable by molecular biology early in the nostrils and saliva; however, antigens are reported later at the onset of symptoms. The lag time in detectable antigen can be critical in the pathogenesis and multiorgan infection. Even though most SARS‐CoV‐2‐infected persons are asymptomatic, around 20% of the patients may have severe manifestations, and 5 to 10% require intensive care. The severe cases with associated mortality are generally older adults with comorbidities; however, severe forms of infection have been detected in all ages. Moreover, virus variants also impact the rate of infection, disease severity and lethality.
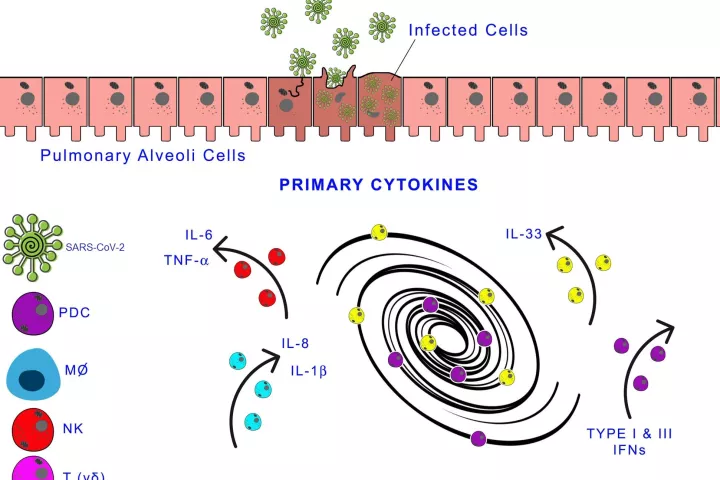
The coronavirus infection causes hypoxemia, from mild to severe (SARS), skin rash, fever, anosmia, fatigue, pain chest, muscle, articular and unexpected hyperglycaemia or increase in blood pressure. The virus affects microcirculation; it generates endothelial cell damage, capillary damage and micro thrombosis. The immune response against the virus and the virus's cytopathic effect induces the activation of the innate immune system, protein and cells, which may generate a massive inflammatory reaction (cytokine storm). The hypoxia induces hypoxia transcription‐inducing factor I (HIF‐I), amplifying the inflammatory response by activating myeloid cells and enhancing transcription of proinflammatory cells and oxidative enzymes. Even though it is still controversial, increased BMI has been considered a risk factor for COVID infection. More studies are required to ascertain the relationship between metabolic syndrome, a subclinical proinflammatory condition and HIF‐I as the triggering factor for massive neutrophil lung recruitment and cytokine storm in SARS‐CoV‐2‐infected patients.
It is unclear how the virus causes neurological effects, and some authors have proposed direct cytopathic effects. Gastrointestinal manifestations, cardiac, kidney and hepatic dysfunctions are observed in human and animals infected by a coronavirus. Skin manifestations reveal immune complex deposition as it has been recorded in other viral diseases, and it is age‐independent. In a general analysis, Mason19 described three phases of SARS‐CoV‐2 viral infection. The first phase is the asymptomatic phase since the infection mostly is present in the nose and on the buccal cavity. In the nose, the immune system involves local antibodies' and innate immune cells that may elicit an adaptative immune response. The induction of the adaptative immune response depends on antigen expression, which is low at the early stages of the viral infection. In the oropharyngeal cavity, the innate immune response is prevalent, complement, neutrophils and macrophages, and antibodies, essentially IgA, bactericidal peptides and enzymes that control mostly bacterial infections. There, the virus elicits a minimal innate immune response. In the moderate symptomatic phase, the virus is primarily present in the larger airways' pseudostratified epithelium. There is an excellent innate immune response in these areas with the recruitment of cells and proteins, which may cause damage and obstruction of the airway. However, the damaged epithelial cells can be removed and replenished with basal cells. There is a more severe disease in the bronchioles where the club cells are usually infected and affect surfactant production and other secretory products. In severe cases, the alveoli are compromised; the virus targets the epithelial type II cells that express ACE. The decrease in viable epithelial type II cells is responsible for respiratory insufficiency. The lack of lung surfactant, alveolar flooding and loss of the extracellular matrix generating more viral infection affects the pulmonary parenchyma's standard repair and the inflammation's resolution. The typical active resorption of alveolar fluid and electrolytes is also hampered, resulting in hypokalaemia. Impaired endothelial cells lead to transudation of plasma protein of inflammatory origin and an irregular formation of hyaline membranes. Residual fibrosis may result after viral infection due to the low resolution of the inflammatory response.
The evidence of lung tissue destruction came from an exciting study analysing proteins of organs of autopsies by HPLC/MS.20 The authors were able to identify high amounts of cathepsin L1, an enzyme involved in intracellular protein catabolism. The increase in cathepsin L1 is related to extracellular matrix degradation critical in viral infection and release.
Significant alterations in blood electrolytes have been described in hypertensive patients infected with SARS‐CoV‐2, hypokalaemia, hyponatraemia and hypocalcaemia. Nonetheless, few studies have dealt with the mechanism involving electrolyte imbalance with neural, cardiovascular, gastrointestinal and renal dysfunctions due to either the general renin‐angiotensin system or the virus's cytopathic effect as it occurs in the lung. Electrolyte imbalance induces inflammasome activation and, consequently, exacerbates the inflammatory response; then, multiorgan dysfunction in severe COVID patients may result from multiorgan‐induced inflammatory reaction. ...
The whole review is available HERE.